Medical power supplies
Leading solutions for medical devices and healthcare equipment
The healthcare manufacturing industry needs to meet an exceptionally high level of safety approvals. Our power supply solutions meet the demanding standards required to ensure patient safety and wellbeing.

Safe, reliable power conversion for medical equipment
Accelerate your time to market with our trusted and proven medical power solutions
It’s vital that medical and healthcare equipment operates with stringent safety mechanisms. An incorrectly specified power converter could lead to lengthened time to market and additional certification costs.
With a deep understanding of the medical sector’s unique power supply needs, we offer a wide range of certified, compact, reliable solutions with robust thermal and EMC design and the opportunity for customization.

EMC challenges during development
Certified medical power solutions for trouble-free integration
We understand that the correct selection and integration of power conversion devices is fundamental to the emissions and immunity performance of your product.
Our healthcare power solutions are fully certified to the latest medical device emissions and immunity standards. Our expertise in power conversion integration enables us to mitigate problems, advising on best practice and our experienced applications engineers can help with any issues.
Reliability is critical in medical devices
Proven, robust power conversion solutions
The correct selection of medical power products is critical to long-term system reliability. A power converter designed to minimize stresses on components during normal operation can still suffer from poor reliability if subject to excessive heat in the end installation.
Good thermal management is essential for maximizing reliability and service life. XP Power offers a selection of products designed for fan-less convection and conduction cooling as well as fan-cooled ratings to suit the requirements of the healthcare application.
Certified power conversion products for medical devices

External power supplies
- Power ranges from 6W to 300W
- Wall mount and desk-top solutions
- Class I and class II for hospital and non-hospital use
- Designed for patient contact applications
- Class II with IP ratings for home-healthcare
AC-DC Power supplies
- Power ranges from 3W to 30kW
- Designed for BF applications
- International medical safety and EMC certification
- Multiple cooling options
- High voltage solutions to 500kV

DC-DC power converters
- Power Options from 1W to 30W
- 1 or 2 MOPP, suitable for BF & CF applications
- International medical safety and EMC certification
- Single and dual outputs
- High voltage solutions up to 25kV
Get in touch
We recognize your need for safe, certified, and highly reliable solutions specifically developed for the healthcare sector. Contact us today for technical support, the latest product samples, up to date pricing and global shipping details.

Custom power solutions tailored to your specific needs
Facing tight project schedules, complex integration, regulatory hurdles and demanding safety standards?
Our expert engineers work closely with your team to develop scalable, application-specific power solutions. With extensive experience in intricate design and a global manufacturing reach, we deliver cost-effective, custom solutions built to the highest standards, ensuring seamless alignment with your design parameters.
We resolve your power supply challenges, allowing you to focus on business priorities.
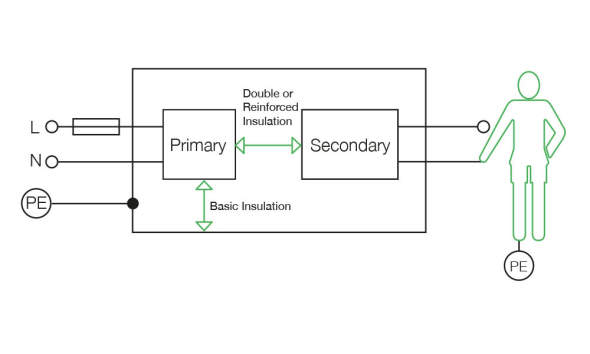
Isolation barriers in medical equipment
We understand the fundamentals of isolation barrier requirements of medical designs
The diagram below illustrates isolation for a typical medical device with an applied part. Two isolation barriers are required to ensure that the applied part is isolated from the ground and meets the patient leakage current limits during normal and single fault conditions. The primary isolation barrier for this type of equipment can be provided by an XP Power supply, while the secondary isolation barrier is provided by another system component or XP solution.
Advanced power converter technology
Leading edge power solutions for medical applications
Backed with exceptional technical support our extensive range of medical AC and DC power solutions ensures there is an exact fit for your healthcare equipment
- Output power from 1W to 5kW
- AC input and DC input solutions
- Solutions with approvals suitable for BF and CF applications
- Fanless solutions for low audible noise and high reliability
- Non-standard solutions for ease of integration
- Defibrillator-proof DC-DC converters
- ISO13485 manufacturing system
- Global manufacturing locations



